Pharmacometrics
Pharmacometrics is the science of quantitative pharmacology and supports efficient drug development and/or regulatory decisions.
Focus on data driven models supporting mainly new drug and formulation development covering small and large molecules.
• Phase I, II, III clinical trials
• Routine clinical data
• Time & Dose dependent PK
• Target-mediated Drug Disposition
• Covariate selection & evaluation
• Sustained release formulations
• Micro- & Nanoparticles
• In vitro-in vivo correlation
Focus on mechanistic-based predictive models supporting basic research and new drug and formulation development covering a wide range of therapeutic areas.
• In vitro, ex vivo & in vivo data models
• Models from combination therapies
• Disease progression and Baseline models
• Delayed continuous and non-continuous responses
• Latent response analysis
• Covariate selection & evaluation
• Phase I, II, III clinical trials
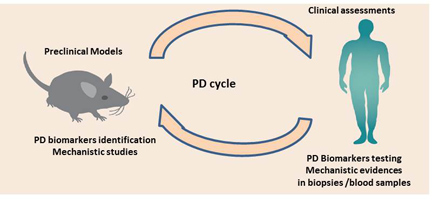
Focus on predicting clinical outcomes using mechanistic models developed at earlier stages, and using the concept of system, disease, and drug action specific parameters.
• Predicting clinical response from pre-clinical PKPD models
• Mechanistic PK and PKPD
• Biomarker response to clinical outcome
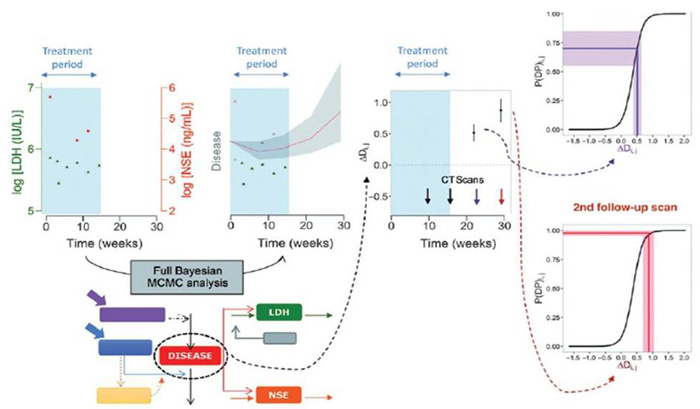
Optimised and improved patient specific therapeutic strategies through the use of population PKPD models using early and limited patient data
• Oncology
• Auto-immune diseases
• Anti-infectives
• Immuno-oncology
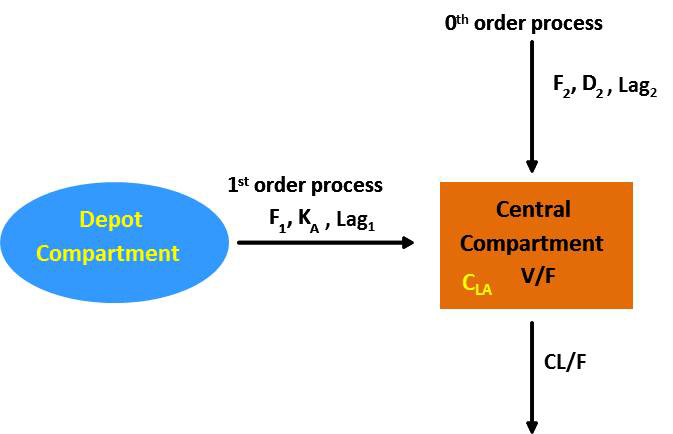
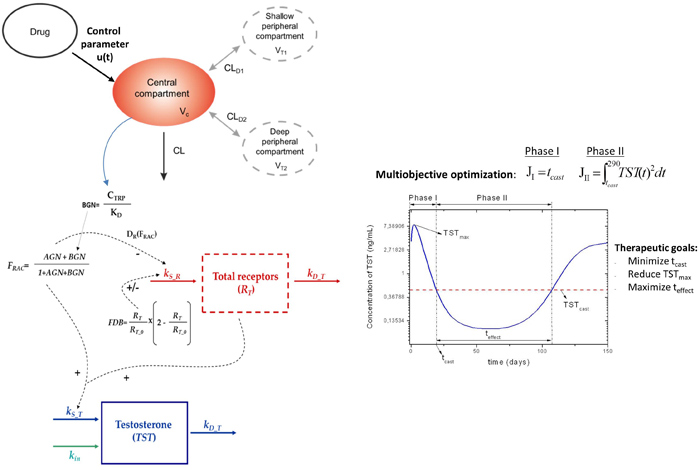
Help designing biopharmaceutics taking into account critical aspects of the response profiles, absorption & pharmacokinetic properties.
• Single- & multi-objectives
• Sustained release formulation
• Time to next administration tim
• Chronic diseases
Systems pharmacology
Systems Pharmacology studies are becoming an increasingly important approach in understanding complex diseases which cannot be fully understood by analysing isolated targets or molecular pathways.
Systems Pharmacology for effIcient Drug Development On R (SPIDDOR), is a methodology to perform Boolean modelling and simulation in the area of Systems Pharmacology and is implemented in R.
SPIDDOR enables (i) model implementation, (ii) simulation of activation profiles of the nodes, (iii) attractor analysis, (iv) a system perturbation and clustering analysis, and (v) convenient graphical summary/display of results.

When quantitative and longitudinal data are scarce, we use a semi-quantitative framework useful to answer drug development questions as target relevance, patient stratification and explorations of beneficial therapeutic combinations.
SP models can be viewed as networks models representing potential pharmacological-target systems as a function of their key elements (genes, proteins or metabolites) denoted by nodes and their interactions.
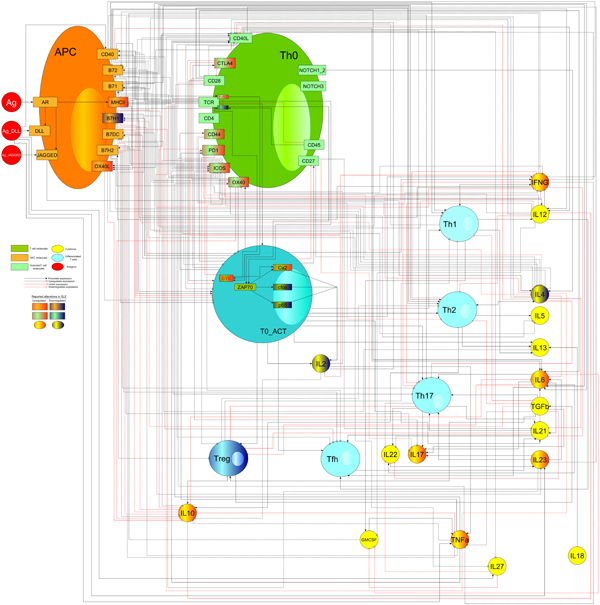
Quantitative description of the longitudinal profiles in complex biological networks allows more precise answers than network based models. Decisions about dosing, required target engagement or formulation requirements can be supported by these models.
Likewise this fully quantitative approach admits a rigorous sensitivity analysis to detect most relevant processes and its relationship with proposed surrogate biomarkers.
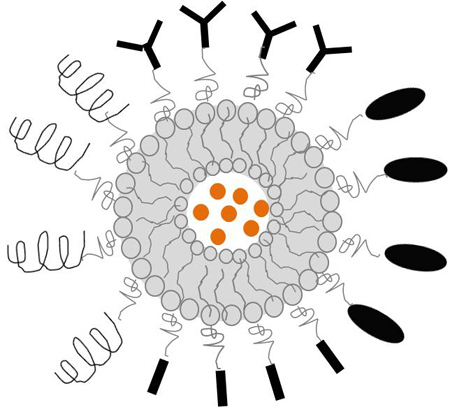
Advanced therapy approach
Development of different targeted nanoliposomes encapsulating anti-tumour agents to apply in cancer animal models.
Nanosize drug delivery systems are overcoming the delivery limitations of many agents at the specific tissues. The versatility of the targeting (including active and passive) is provided by attaching at the nanosystem surface different types of ligands able to recognise antigens or receptors specifically expressed or up-regulated in tumour cells. Additionally, these nanocarriers present a controlled release property to achieve an increase therapeutic efficacy and reduce side effects.
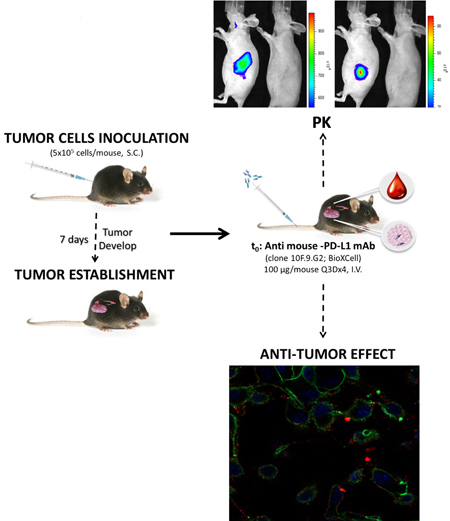
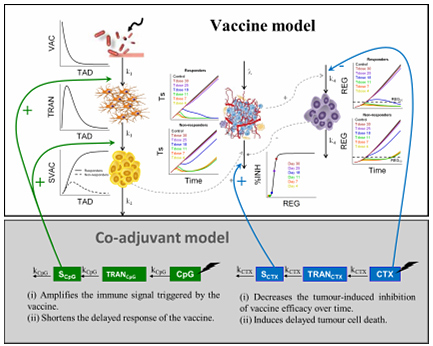
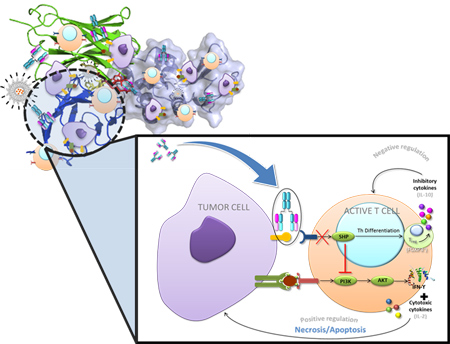
In vivo evaluation of immune-modulator agents (mAbs, vaccines and peptides).
The stimulation of an antitumor immune response applying biomolecules is one of the most relevant aim pursued by many researchers, in the last decades. Immune checkpoints represent one of the mechanisms used by the tumours for the evasion of this immune system. The blockade of these immune checkpoints using specific monoclonal antibodies has demonstrated clinical benefit in several types of cancers (melanoma, squamous non-small cell lung cancer, bladder cancer…). The characterisation of several biomarkers involved in these mechanisms might throw some light on the possible combination of therapies to attain the desired effect. In that sense, other strategies such as vaccines or certain peptides are being used to seek the immune system stimulation.
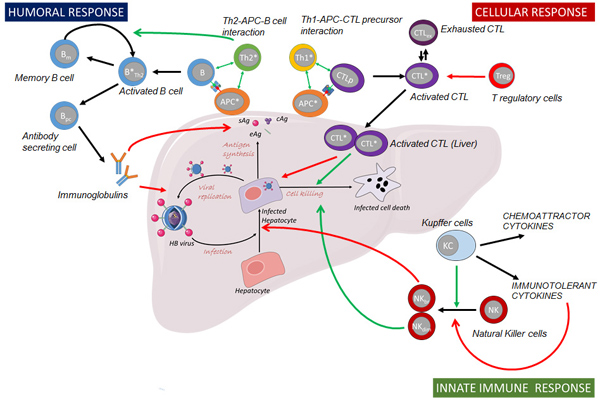
Application of in vitro/ in vivo platform to develop PKPD mechanistic models.
Preclinical models are the previous requirement to develop a therapeutic agent. The first step to characterise in a quantitative way the pharmacokinetics and pharmacodynamics relationship for a specific agent is totally necessary to use animal models. These studies provide relevant insight about the agent behaviour at the organ level, which combined with the culture cell experiments explain in detail some processes that take place at intra- and cellular level. Therefore, all of these data provide us with essential information for the development of mechanistic-based models able to describe and predict those responses induced by the treatment.